
For galvanic anodes the value of V is fixed and less than 1 Volt thus the amount of current that can be generated from a galvanic anode is also limited. The system resistance is in large part dictated by the environment and not easily changed.
Higher Current Systems. The basic formula for a DC circuit is that V = I x R (Ohm’s law) where V is the voltage difference or driving force, I is the system current and R is the system resistance. Today’s most efficient anodes are the dimensionally stable mixed metal oxide anodes (MMO.) These anodes have exceptionally long anode life relative to other anode choices. Longer Life Anodes. Because the choice of anodes is not dependent on the electrical potential of the anode itself, impressed current anodes can be selected based on other factors such as anode material cost, current density and consumption rates. Why Impressed Current Cathodic Protection (ICCP)? The use of an external power supply enables an impressed current system to generate significantly higher current output with fewer, longer lasting anodes than any sacrificial anode system. Impressed current anode systems are different than galvanic (sacrificial) systems because they utilize an external DC power supply to create the electrical current flow. Typical galvanic anodes include magnesium, zinc and aluminum as each of these are more electrically negative than carbon steel or other steels. This current flow results in a rapid consumption of the anode, hence the common term “sacrificial anode” is often used to describe these anode systems. Galvanic anode systems (also termed sacrificial anode systems) use a metal that is naturally more negative than the metal being protected and thus when the two metals are connected electrically to each other, current flows from the metal that is more electrically negative to the metal that is more electrically positive. There are two basic types of CP systems: galvanic (or sacrificial) and impressed current. Unfortunately, atmospherically exposed metal cannot be cathodically protected because air is not a conductor of electrical current, but most submerged and buried applications are suitable for cathodic protection including pipelines, ships, docks, jetties, storage tanks, and a range of other structures. Cathodic protection is used to prevent corrosion in a wide range of applications where the structure being protected is surrounded by an environment that allows current flow. When properly designed and applied, cathodic protection systems stop the corrosion process. This protective current changes the environment around the metal thus halting the corrosion reaction. 
The Corrosion ProblemĬorrosion of metals is a naturally occurring electrochemical process that causes the metal to oxidize and deteriorate when exposed to the environment (commonly referred to as rusting.) Cathodic protection (CP) is a means to prevent corrosion by applying a flow of electrical current from an external source (anode) through the environment and on to the metallic structure that is being protected.
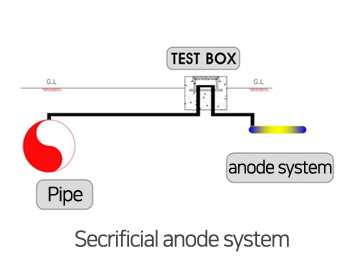
This makes it possible to protect virtually any structure, regardless of size or current requirements using long life anodes and enough appropriately sized power supplies. Impressed current cathodic protection systems have the benefit of using an external power supply to drive current.






 0 kommentar(er)
0 kommentar(er)
